LuViva is intended for use after…
- abnormal cytology and/or positive HPV findings and/or other risk factors
- to triage women aged 16+ for additional evaluation prior to colposcopy and biopsy
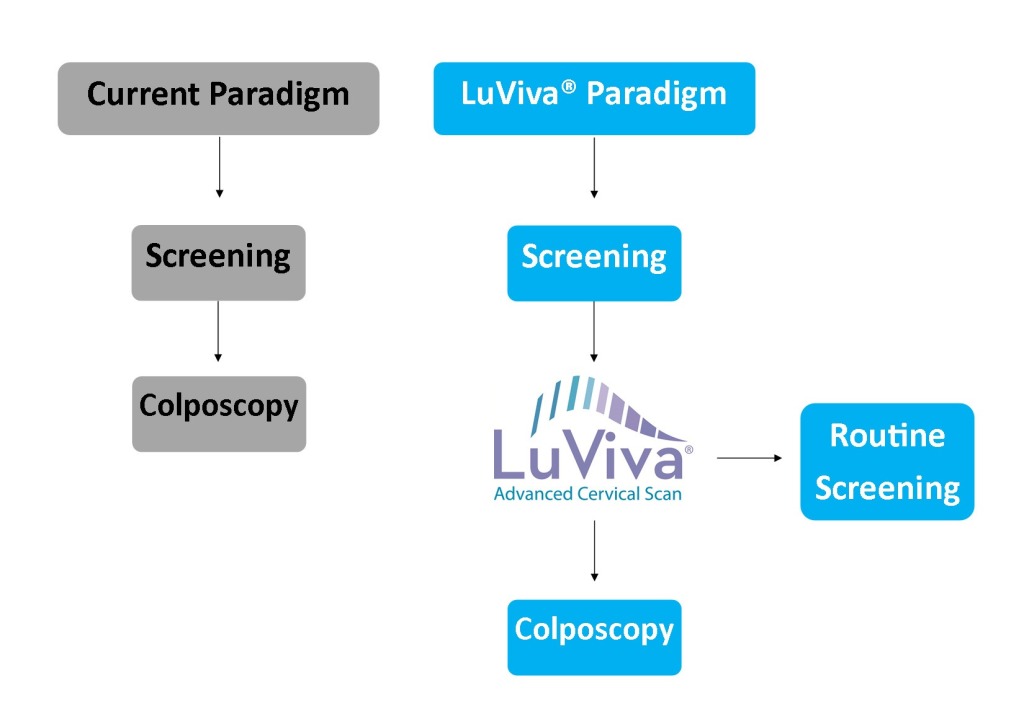
LuViva is to be used on a referred population of women following an abnormal pap test, positive HPV test, or other symptomatic referral cause. The current cervical cancer screening paradigm has 100% women who have received an abnormal results from their initial cervical cancer screening method go onto diagnostic testing, which can include HPV testing, colposcopy, and/or biopsy. When adding LuViva to the cervical cancer screening process, LuViva acts as a triage point identifying women that can return to routine screening from those that should go onto further diagnostic testing. Based on clinical trials, 20% of women being referred onto additional testing will actually have disease. Of the remaining 80%, LuViva would be able to remove 35-40% of them from additional, unnecessary testing.
Contraindications
- Pregnancy
- Menstruation on the exam day
- Radiation therapy to the genitourinary system within a year of the exam
- Prior hysterectomy
- Congenital anatomic cervical variant (eg, double cervix)
- Friable cervix
- Postcoital or other significant bleeding
- Excessive cervical mucus or discharge that cannot be removed or the operator believes is significant enough to interfere with a cytology or colposcopy
- History of photosensitivity or other disease affected by ultraviolet radiation
- Phototherapy
- Recent use of photosensitizing agents
- Referral test indicating risk of severe dysplasia