Cervical Cancer Detection
Globally, cervical cancer is the second-most common cancer in women.1 Typically, an abnormal Pap test (cytology) or human papillomavirus (HPV) test is followed up with a colposcopy exam and biopsy.2–4 Early detection with these tests has reduced cervical cancer mortality.1 The tests may, however, produce 2–4
- False-positive results, leading to additional, unnecessary tests and procedures, increased institutional costs, and patient stress and anxiety
- False-negative results, leading to delays in disease diagnosis
Introducing LuViva® Advanced Cervical Scan
LuViva is a point-of-care device using fluorescence and reflectance spectroscopy to scan the entire uterine cervix for detecting moderate to high-grade cervical dysplasia (cervical intraepithelial neoplasia [CIN] 2 or higher). LuViva is used before colposcopy or indicated as a follow-up or recall procedure to triage the disease state of women with an abnormal cytology or other risk factors.
LuViva Is Recommended for Women 16 Years of Age or Older Who:
- Have abnormal cytology or positive HPV findings
- Have other risk factors (eg, previous dysplasia)
- Need additional evaluation before colposcopy or biopsy
Benefits and Risks of LuViva®
- Is noninvasive and fast (real-time results in about a minute)
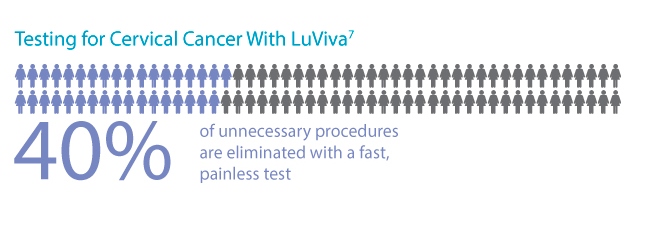
- May reduce the number of women with normal cervices or low-grade disease having to undergo colposcopy or biopsy5
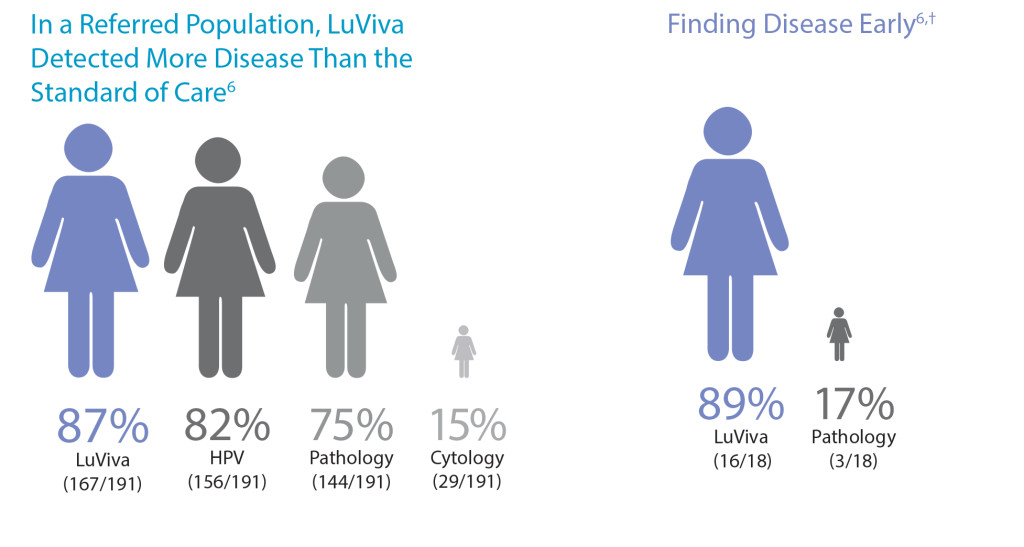
- May increase the detection of CIN2+ disease missed by other tests and procedures5
- Does not require interpretation by an outside laboratory